Promotional Features
HMOs: A prebiotic fostering a resilient family foundation across all life stages
Human milk oligosaccharides (HMOs) are naturally occurring components of human breast milk that act as prebiotics.
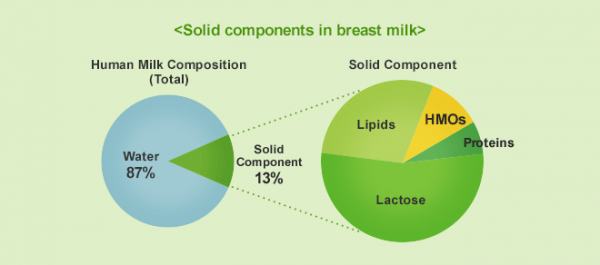
HMOs are the third most abundant solid component in human breast milk, after lactose and lipids (Figure 1). Factors such as genetics, ethnicity and geographical location can affect the composition of HMOs in each individual.1 HMOs are present at levels of 20-25 g/L in colostrum and generally found within a range of 5-20 g/L in mature breast milk.2
Approximately 200 types of HMOs have been detected of which 168 types have been structurally determined.3 Most HMOs have lactose-bound galactose, fucose, N-acetylglucosamine, and N-acetylneuraminic acid (sialic acid). HMOs are generally classified according to the property of saccharides, categorizing them as either neutral HMOs and acidic HMOs respectively.
Among neutral HMOs, 2’-fucosyllactose (2’-FL) consists of L-fucose, D-galactose and D-glucose. This is the most abundant HMO naturally present in human breast milk, making up about 30% of all of HMOs.4 Sialyllactose (SL), consisting of sialic acid and lactose, is an acidic HMO linked to healthy infant growth, notably 3’-SL and 6’-SL, which are major forms of sialic acid in breast milk.5
In infants, many HMOs reach the large intestine without being digested in the small intestine, thereby stimulating the growth of valuable gut bacteria.6 Since HMOs have been detected in the urine and plasma of breast-fed infants, some of them are absorbed and are thought to circulate through the body to exert their physiological functions.7,8 HMOs have also been detected in the amniotic fluid of pregnant women, suggesting that foetuses may be exposed to HMOs in the womb.9
Breastfeeding is recognised as the gold standard of infant nutrition by the World Health Organization (WHO) and it is suggested that these indigestible oligosaccharides, specifically found in human breast milk, could be linked to prebiotic, immunological, anti-pathogen properties, as well as gut and intestinal health properties, thereby supporting healthy infant growth.1,10
Figure 1: Solid components in breast milk. HMOs are the third most abundant solid component in human breast milk, after lactose and lipids.
HMOs are complex carbohydrates for infants’ brains and can naturally support the immune system
Interventional trials in infants report that 2’-FL and lacto-N-neotetraose promote the growth of beneficial bacteria in the gut, such as Bifidobacterium, and are involved in shaping the infant gut microbiota and modulating immune function.11 In vitro and animal studies have suggested anti-infective effects against viruses and bacteria, alleviation of food allergic symptoms, prevention of necrotizing enterocolitis (NEC), acceleration of intestinal maturation, metabolic regulatory functions, and involvement in the development of cerebral function in infants.2,12,13 It is becoming clear that the effect manifested by the type of HMO also differs.
Several observational or preclinical studies suggest potential contribution of sialyllactose to brain health. One observational study shows 6’-SL content in breast milk was positively correlated with cognitive development of babies assessed by Bayley-III scales at six and 18 months of age.14 Another observational study showed that 3’-SL content in breast milk was positively correlated with language development in babies, using the Mullen Scales of Early Learning (MSEL) as the primary outcome measure to assess early cognitive development.15
The composite score and 3’-SL levels were positively associated in a sub-group analysis. This association was driven by the receptive and expressive language subdomain scores. Furthermore, there was an interaction between 3’-SL and age for receptive language.
HMOs for all ages
An in vitro study suggests the importance of a mixture of HMOs for immunity. Four milk oligosaccharides – 2’-FL, 3’-SL, 6’-SL, and galacto-oligosaccharides – were tested for their effects on the infectivity of human rotaviruses G1P[8] and G2P[4].16 When compared with infections in the absence of glycans, all oligosaccharides substantially reduced the infectivity of both human rotavirus strains.
A mixture of 3’-SL and 6’-SL, at the same ratio as that in breast milk, was more potent in reducing G2P[4] infectivity (73% reduction, P < 0.01) compared with 3’-SL (47% reduction) or 6’-SL (40% reduction) individually. Another SHIME® system study, using microbes obtained from three healthy adults, also suggests that 3’-SL and 6’-SL could act as a unique prebiotic in an adult gastro-intestinal microbiome environment.17
World’s first invention for large-scale production of HMOs by fermentation
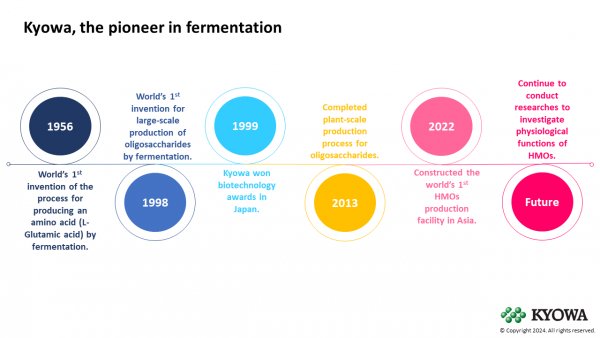
Since 1956, Kyowa has been the pioneer in cutting-edge technologies of producing beneficial chemicals by means of fermentation. In 1998, Kyowa established a novel production technology for oligosaccharides and sugar nucleotides, the world’s first invention to make large-scale production of oligosaccharides possible by fermentation (Figure 2).18
Figure 2: Kyowa established the world’s first invention for large-scale production of oligosaccharides through fermentation and the world’s first HMO production facility in Asia.
Because HMOs are present only at lower levels in other mammals, such as cattle or goats, their extraction from nature is limited in both type and quantity. Various manufacturing methods of HMOs, including chemical or enzymatic synthesis have been explored. However, these methods are time-consuming, produce lower yields and are less cost-efficient to realize large-scale production of high-quality HMOs at low-cost.
As HMOs contain many hydroxyl groups, it is challenging to bind multiple sugars in a positional and stereoselective manner.18 Therefore, an enzymatic method via industrial fermentation with E. coli to produce HMOs has been established.
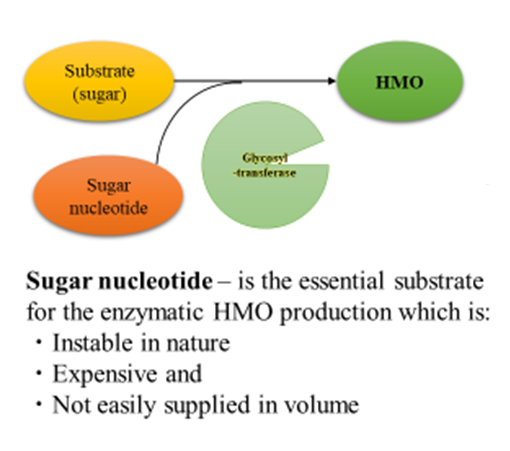
The need for sugar nucleotides, which are high-energy compounds, as substrates for glycosyltransferases has been a long-standing challenge for industrialization. However, a technology has since been established that allows microorganisms to produce sugar nucleotides from inexpensive carbon sources, such as glucose. The method of transferring sugar itself using glycosyltransferase and adding lactose is currently the mainstream method.18,19
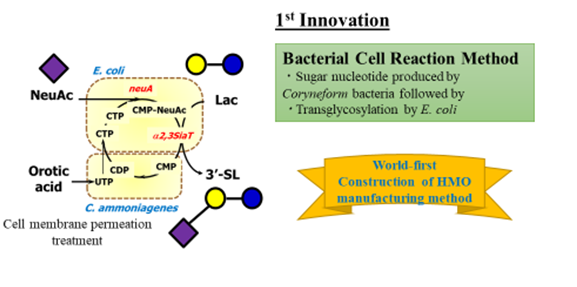
Production of both sialyllactose and fucosylated oligosaccharides require an appropriate enzyme glycosyltransferase and the combination of an appropriate ATP regenerative system. Appropriate glycosyltransferase allows multiple sugars to bind to the correct positions through transferring lactose by using activated glyco nucleotides (sugar nucleotides) as substrates (Figure 3). Kyowa established two methods to produce HMOs: the bacterial cell reaction method and the direct fermentation method.
The bacterial cell reaction method is designed to produce sugar nucleotide in one bacteria and to connect with another bacteria to trans glycolate, avoiding the issue of instability and supply of sugar nucleotide. The sugar nucleotides production system was established by combining recombinant E. coli expressing genes involved in sugar nucleotide biosynthesis and Corynebacterium to form nucleoside 5’-triphosphate.
This technology was the first in the world to mass produce HMOs (Figure 4). The direct fermentation method was created later to complete these processes in one single bacteria, further improving the production efficiency (Figure 5).
Figure 3: Schematic flow of enzymatic HMO production with sugar nucleotide.
Figure 4: Production of 3’-sialyllactose by bacterial cell reaction method.
Figure 5: Production of sialyllactose by direct fermentation.
The ground-breaking innovation to mass-produce HMOs through fermentation stands as a testament to the transformative potential of science in enhancing human health and wellbeing. While the diverse landscape of HMOs offers promising avenues for infant development, emerging research indicates potential benefits for wellness across all ages, in various forms of applications.
References
1. Global Prebiotic Association. Prebiotic Type Spotlight: Human Milk Oligosaccharides (HMOs).
2. Bode L. (2012). Human milk oligosaccharides: every baby needs a sugar mama. Glycobiology, 22(9), 1147–1162.
3. Ninonuevo, M. R.; Park, Y.; Yin, H.; et al. (2006). A strategy for annotating the human milk glycome. Journal of agricultural and food chemistry, 54(20), 7471–7480.
4. Vandenplas, Y.; Berger, B.; Carnielli, VP.; et al. (2018). Human Milk Oligosaccharides: 2′-Fucosyllactose (2′-FL) and Lacto-N-Neotetraose (LNnT) in Infant Formula. Nutrients. 10(9):1161.
5. Q, Xie.; Y, Xu.; W, Zhang.; et al. (2022). Concentration and distribution of sialic acid in human milk and its correlation with dietary intake. Frontiers in Nutrition. Vol 9.
6. Newburg, D.S.; (1996). Oligosaccharides and glycoconjugates in human milk: Their role in host defense. J Mammary Gland Biol Neoplasia 1, 271–283.
7. Goehring, K. C.; Kennedy, A. D.; Prieto, P. A.; et al. (2014). Direct evidence for the presence of human milk oligosaccharides in the circulation of breastfed infants. PloS one, 9(7), e101692.
8. Marriage, B. J.; Buck, R. H.; Goehring, K. C.; et al. (2015). Infants Fed a Lower Calorie Formula With 2'FL Show Growth and 2'FL Uptake Like Breast-Fed Infants. Journal of pediatric gastroenterology and nutrition, 61(6), 649–658.
9. Jantscher-Krenn, E.; von Schirnding, L.; Trötzmüller, M.; et al. (2022). Human Milk Oligosaccharides Are Present in Amniotic Fluid and Show Specific Patterns Dependent on Gestational Age. Nutrients. 14(10):2065.
10. World Health Organization. Breastfeeding.
11. Berger, B.; Porta, N.; Foata, F.; et al. (2020). Linking Human Milk Oligosaccharides, Infant Fecal Community Types, and Later Risk To Require Antibiotics. MBio, 11(2), e03196-19.
12. Urashima, T.; (2014). Applied Glucose Science. Journal of the Japanese Society of Applied Glucose Science. 4, 273.
13. Urashima, T.; Fukuda, K.; (2019). [Review] Advanced Studies of the Biological Functions of Milk Oligosaccharides (4). Bulletin of Applied Glycoscience. Volume 9, Issue 4, Pages 254-265.
14. E, Oliveros.; Martin M. J.; Torres-Espinola, F. J.; et al., (2021). Human Milk Levels of 2´-Fucosyllactose and 6´-Sialyllactose are Positively Associated with Infant Neurodevelopment and are Not Impacted by Maternal BMI or Diabetic Status. J Nutr Food Sci. 4: 024.
15. Cho, S.; Zhu, Z.; Li, T.; et al. (2021). Human milk 3'-Sialyllactose is positively associated with language development during infancy. The American journal of clinical nutrition, 114(2), 588–597.
16. Laucirica, D. R.; Triantis, V.; Schoemaker, R.; et al. (2017). Milk Oligosaccharides Inhibit Human Rotavirus Infectivity in MA104 Cells. The Journal of nutrition, 147(9), 1709–1714.
17. Sato, Y.; Kanayama, M.; Nakajima, S.; et al. (2024). Sialyllactose Enhances the Short-Chain Fatty Acid Production and Barrier Function of Gut Epithelial Cells via Nonbifidogenic Modification of the Fecal Microbiome in Human Adults. Microorganisms. 2024; 12(2):252.
18. Endo, T.; Koizumi, S.; Tabata, K.; et al. (2000). Large-scale production of CMP-NeuAc and sialylated oligosaccharides through bacterial coupling. Applied microbiology and biotechnology, 53(3), 257–261.
19. Ujihara, T.; et al., (2022). Glycoforum. 25, A7J.